Vibrio
History:
During the 19th century ,cholera spread across the world wide.The current pandemic started in South Asian in 1961 after reached Africa in 1971and the America's in 1991.
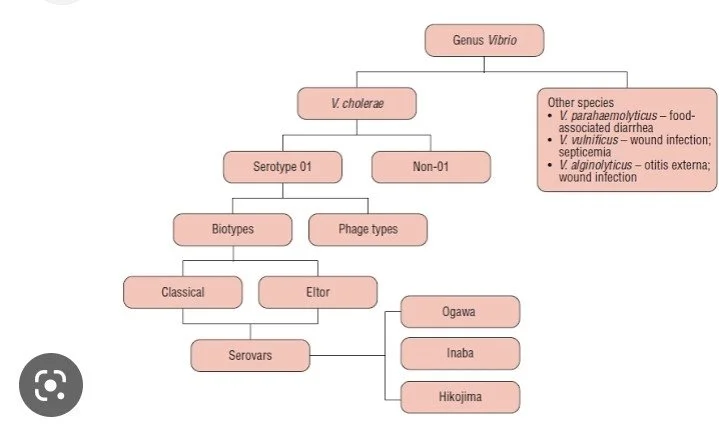
Scientific classification
Domain:Bacteria
Phylum:pseudomonadota
Class:Gammproteobacteria
Order:vibrionales
Family:vibrionaceae
Genum:vibrio
Morphology:
* vibrio is a genus of gram negative curved bacilli.
*They are actively motile by means of polar flagellum.
*The name vibrio is derived from its characteristics vibratory motility
(From vibrare meaning 'to vibrate')
* vibrios are present in marine environments and surface waters worldwide.
*The most important member of the genus is vibrio cholerae.
* The vibrio cholerae is a short curved rods,about 1.5×0.2_0.4MU m.
*pleomorphism is common in old culture.
*In stained films of mucus flakes taken from acute cholerae cases,the vibrios are seen arranged in parallel rows this is described as a' Fish in a stream' appearance.
Classification:
Based on their requirement of sodium chloride, vibrios are classified as halophilic and non- halophilic .
*Halophilic: v.parahemolyticus ,v.alginolyticus,v.vulnificus.
*Non-halophilic: vibrio cholerae
Resistance:
* vibrio cholera are susceptible to heat,drying and acids but resist high alkalinity.
*They are killed at 55° c in 15 minutes.
*In clean tap water they survive for thirty days .
* In untreated soil,they may survive for several days.
* On fruits, they survive for 1-5 days at room temperature and for a week in the refrigerator.
*vibrio cholera killed in gastric juice with in few minutes.
Symptoms:
* high fever
* weight loss
*feeling of nausea
* bloating in the belly
*blood pressure becomes low
Adapted from: public health notes.com
Pathogenesis:
* The vibrio cholera remain in the gut and does not multiply into the blood stream.
*It adheres to the mucosa of the small intestines by both outer membrane protein and flagella adhesions.
*vibrio cholera produces enterotoxin that causes excessive fluid and electrolyte loss.
*sodium chloride aborsorption is inhibited and therefore excreted resulting in water ,sodium chloride and potassium bicarbonate loss.
Lab diagnosis:
Specimen collection and transport
* Stool specimens suspected of containing vibrio species should be collected and transported only in the cairy Blair medium.
*Buffered glycerol medium is not acceptable because in vibrio glycerol is toxic.
*Feces is preferable ,but rectal swabs are acceptable during the acute phase of diarrheal illness.
Direct Detection Method
*vibrio cholera toxin can be detected in stool using an enzyme linked assay (ELISA)or latex agglutination test.
* when stool specimens are examined using a dark field microscopy , the bacilli exhibit characteristics rapid darting ( or) shooting star motility
Treatment:
* Rehydration
- IV lactated Ringer's ( L R)
-ORS : by mouth (or) by N G tube
-Zinc supplement
* Antibiotics
Adults : Deoxycycline 300 mg PO×1
Children or pregnant women: TMP-SMX ×3 days
Adapted from: Hind pharma
Prevention:
*Drink boiled water.
* Avoid consumption of raw foods.
*Avoid dairy products as much as possible .
*Wash your hands with soap.
* Wash fruits and vegetables before you eat.
* Drink plenty of water.
References :
1. Ananthanarayan and Paniker's textbook of microbiology
2.vibrio A presentation by Dr ALPANA VERMA International medical and Technological University, Tanzania
3.cholera By JAMES NYIRENDA
ADENOVIRUS
ADENOVIRUS
by Mr Prithiv Saran B, I MSc Clinical Virology
HISTORY
Rowe and colleagues was the first identified the adenovirus in 1953.
Yes viruses are dangerous but not all the time some virus are useful such as adenovirus is used for gene therapy.
DO YOU KNOW WHY THEY CALLED ADENOVIRUS?
The virus was identified from the adenoid tissue in 1953 by Rowe.
REALM: Varidnaviria
KINGDOM: Bamfordvirae
Phylum : Preplasmiviricota
CLASS : Tectiliviricetes
ORDER : Rowavirales
FAMILY: Adenoviridae
GENERA
• Atadenovirus
• Aviadenovirus
• Ichtadenovirus
• Mastadenovirus
• Siadenovirus
So commonly more than 100 humans adenovirus types have been isolated. And they are classified into seven species (A-G)
A -12,18,31
B-3,7,11,14,16,21,34,35
C- 1,2,5,6
D- 8-10, 13,15,17,19,20,22-30,32,33,36-39,42-47
E- 4
F- 40,41
G- 52
MORPHOLOGY
SHAPE AND SIZE
The size of the adenovirus is 70-75 nm. Then they have a capsid and that capsid is composed of 252 capsomers arranged as icosahedron with 20 triangular facets and 13 vertices.
CAPSOMERS
So finally they have a 252 capsomers, in this 252 capsomers -240 capsomers have 6 neighbours and they are called hexons, while the 12 capsomers at the vertices have only 5 neighbours and they are called pentons. And virion look like as space vehicle.
ENVELOPE
Non envelope (Naked) Virus.
Adapted from Rafie k et al.,(8 Jan 2021)
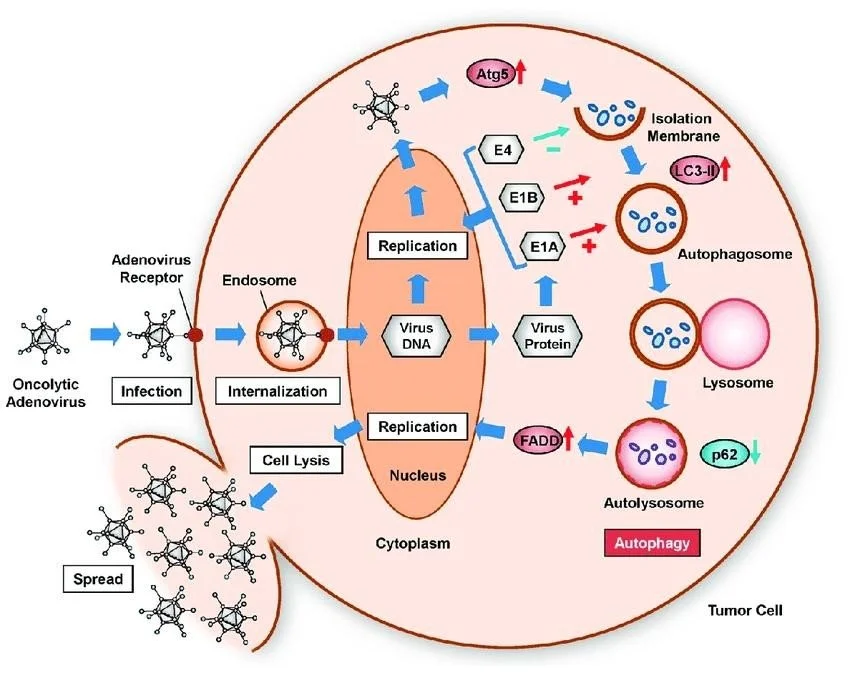
REPLICATION
• Now we are seen about some interesting part , that is replication of Adenoviruses they possess a linear Double stranded DNA genome and are able to replicate in the nucleus of vertebrate cells using the host’s replication machinery.
• After this the adenovirus entry into the host cell involves two sets of interactions between the virus and the host cell.
• Most of the action occurs at the vertices. Entry into the host cell is initiated by the knob domain of the fiber protein binding to the cell receptor.
• The two currently established receptors are: CD46 for the group B human adenovirus serotypes and the coxsackievirus/adenovirus receptor (CAR) for all other serotypes.
Adapted from tazawa et al.,(2017)
• They are currently identified 2 receptors CD46 for the group B adenovirus serotype and Coxsackievirus or adenovirus receptor (CAR) for all other serotypes.
EPIDEMIOLOGY
Two sources of transmission is here 1. Human, 2. Animal
HUMAN
• Adenovirus infection in humans they are broadly caused by types B, C, E and F.
• Transmission: Close contact with another person, fecal–oral route, airborne transmission , small droplets, contaminated surfaces.
ANIMAL
Bat adenovirus TJM ,Squirrel adenovirus (SqAdV), Adenovirus in reptiles.
Cause: Adenoviruses are also known to cause respiratory infections in horses, cattle, pigs, sheep, and goats.
PATHOGENICITY
• Adenovirus Cause infections :
• Respiratory tract
• Eye
• Bladder
• Intestine
PORTAL OF ENTRY
Respiratory tract, GIT, Conjunctivitis Etc
COMMON SYNDROMES Related With Adenovirus Infections
SYNDROME Principal serotype
Respiratory disease in children 1,2,5,6
Sore throat, febrile cold, pneumonia 3,4,7,14,21
ARD in military recruits 4,7,21
Follicular (Swimming pool) conjunctivitis 3,7
Epidermic keratoconjunctivitis 8,19,37,
Diarrhea 40,41
Pneumonia, hepatitis, colitis, etc., In transplant recipients. 34,35
Sarcoma in baby hamster 12,18
Tumours in animals 12,18,31
Transformation of cell culture All type
Human cancer No produce
SIGNS AND SYMPTOMS
Wide range illness
o Common cold or flu-like symptoms
o Fever
o Sore throat
o Acute bronchitis (inflammation of the airways of the lungs, sometimes called a “chest cold”)
o Pneumonia (infection of the lungs)
o Pink eye (conjunctivitis)
o Acute gastroenteritis (inflammation of the stomach or intestines causing diarrhea, vomiting, nausea and stomach pain)
Less common symptoms
o Bladder inflammation or infection
o Neurologic disease (conditions that affect the brain and spinal cord)
LABORATORY DIAGNOSIS
• In very serious case It’s necessary to do lab diagnosis but not necessary in acute stage.
• Sometimes immunocompromised individuals can shed the virus and show no symptoms.
Including diagnosis
• Tissue culture
• Serodiagnosis
• Immunofluorescence
• PCR
TREATMENT
In the case of scenario there is no specific treatment or antiviral drugs to treat adenoviral infections. Most Adenovirus Infections are mild and may require only care to help relieve symptoms. Eg. Paracetamol for fever.
VACCINE
THERE IS ANY VACCINE FOR ADENOVIRUS?
• The answer is yes and also No.
• Vaccine is only available for U.S MILLITARY (1971-1999), It will prevent most illness caused by these two virus types. There is currently no adenovirus vaccine available to the general public.
Vaccine type: Adenovirus 4 and 7. Live virus and it is oral administration.
Adapted from U.S MILLITARY or DEPARTMENT OF DEFENCE EMPLOYEES.
Why only for U.S MILLITARY?
The vaccine is approved for military personnel 17 - 50 years of age. It is suggested by the Department of Defense for military recruits entering basic training. Otherwise at risk of developing ARD from adenoviruses they may use.
ADVERSE EFFECTS
· Headache
· Upper respiratory tract infection
· Abdominal pain
· Nausea
· Diarrhea
· Fever
· Joint pain
· Haematuria
· Pneumonia
· Inflammation in stomach
· Etc,.
PREVENTION
Simple steps we have to follow that is enough to protect our self
· Wash your hands with soap at least for 20 sec.
· Avoid touching your eye, nose, mouth with unwashed hand.
· Avoid close contact with people who are infected.
If your infected you can also help protect others
· Stay home when your are infected
· Cough and sneeze into your tissue not in your hand
· Avoid close contact with people
· Avoid sharing your food and water to others
REFERENCES
Tazawa H, Kuroda S, Hasei J, Kagawa S, Fujiwara T. Impact of Autophagy in Oncolytic Adenoviral Therapy for Cancer. Int J Mol Sci. 2017 Jul 10;18(7):1479. Doi: 10.3390/ijms18071479. PMID: 28698504; PMCID: PMC5535969.
Ewer KJ, Lambe T, Rollier CS, Spencer AJ, Hill AV, Dorrell L (August 2016). “Viral vectors as vaccine platforms: from immunogenicity to impact”. Current Opinion in Immunology. 41: 47–54. Doi:10.1016/j.coi.2016.05.014. PMID 27286566. S2CID 12661335.
Rafie K, Lenman A, Fuchs J, Rajan A, Arnberg N, Carlson LA. The structure of enteric human adenovirus 41-A leading cause of diarrhea in children. Sci Adv. 2021 Jan 8;7(2):eabe0974. Doi: 10.1126/sciadv.abe0974. PMID: 33523995; PMCID: PMC7793593.
W. S. M. Wold, M. G. Ison, Adenoviruses, in Fields Virology, D. M. Knipe, P. M. Howley, Eds. (Lippincott Williams & Wilkins, 2016), vol. 2, chap. 56, pp. 1732–1767.
M. Garofalo, M. Staniszewska, S. Salmaso, P. Caliceti, K. W. Pancer, M. Wieczorek, L. Kuryk, Prospects of replication-deficient adenovirus based vaccine development against SARS-CoV-2. Vaccines 8, 293 (2020).
Tucker SN, Tingley DW, Scallan CD. Oral adenoviral-based vaccines: historical perspective and future opportunity. Expert Rev Vaccines. 2008 Feb;7(1):25-31. Doi: 10.1586/14760584.7.1.25. PMID: 18251691.